Research
Chitin, a linear polymer of N-acetyl-b-D-glucosamine (GlcNAc), is the most abundant amino
biopolymer in nature. In particular, it is found in arthropod exoskeletons and
fungal cell walls, but also in other major Phyla such as annelids, mollusks and
coelenterates (Wagner, 1994). Since chitin is usually not found in vertebrates
(except for bony fishes) and plants, enzymes involved in its synthesis
constitute ideal targets for environmentally safe and selective agents used to
control fungi-born diseases and insect pests.
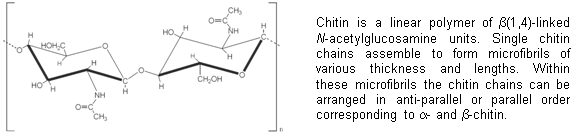
Chitin synthesis is catalyzed by the enzyme chitin
synthase (CHS) (EC 2.4.1.16), a large integral membrane protein that belongs to
the family of b-glycosyltransferases. Most of the current knowledge
on these important enzymes originates from studies performed in fungi. However,
there are still many unsolved questions to answer, mainly due to the lack of
structural and functional data.
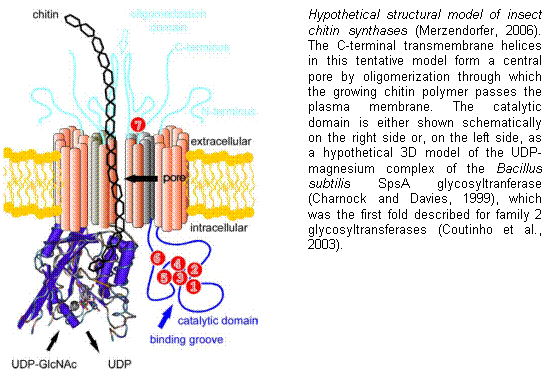
In comparison to
fungal systems, very little is known about insect CHSs, even though chitin
synthesis is essential for growth and development. However, recent advances in the
molecular biology of insect CHSs have taken a big step towards a better
understanding of these important enzymes. During the last years, CHS genes and
cDNAs have been cloned from several species including dipteran insects like Lucilia cuprina (Tellam et al., 2000), Aedes aegypti (Ibrahim et al.,
2000) and Drosophila melanogaster (Gagou et al.,
2002), the lepidopteran Manduca sexta (Zhu et al., 2002) and the
coleopteran Tribolium castaneum (Arakane et al., 2004).
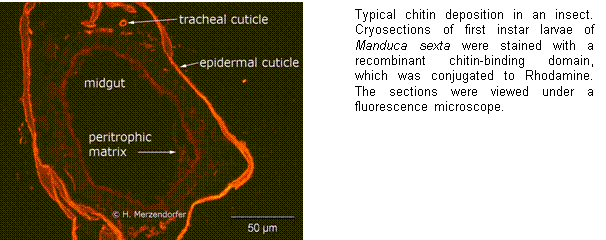
Insect CHSs appear to be encoded by only
two genes, CHS-A and CHS-B, as deduced from Drosophila, Anopheles
and Aedes genome resources as well as from genomic Southern blots (Hogenkamp et al., 2005; Arakane et al., 2004; Merzendorfer
and Zimoch, 2003; Zimoch and Merzendorfer, 2002). Gene expression studies
performed in Lucilia, Tribolium and Manduca indicate that CHS-A is specifically
expressed in the epidermis and related ectodermal cells such as tracheal cells,
while the expression of CHS-B has been suggested to be specific for gut
epithelial cells that produce chitin in the context of peritrophic matrix (PM)
formation (Arakane et al., 2004; Lehane, 1997;
Merzendorfer and Zimoch, 2003; Merzendorfer, 2006;Tellam
et al., 2000). Most of the studies performed on chitin synthesis in insects so
far have focused on the ectodermal isoform expressed by epidermal cells. Since
the PM has pivotal functions for insects, we are also interested in chitin
synthesis by midgut epithelial cells, which we analyze with biochemical,
biophysical and immunological methods using different insect models: Manduca sexta, a pest of solanaceous plants, the
yellow fever mosquito Aedes aegypti,
a vector for animal and human diseases, and the red flour beetle Tribolium castaneum, an pest
of stored
agricultural products, as
insect models. We examine the involved processes with biochemical and genetic
methods (including transgenic animals, RNA interference).
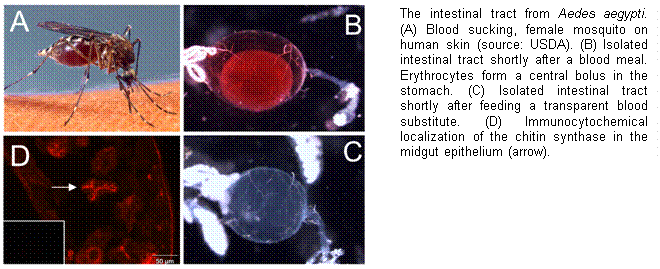
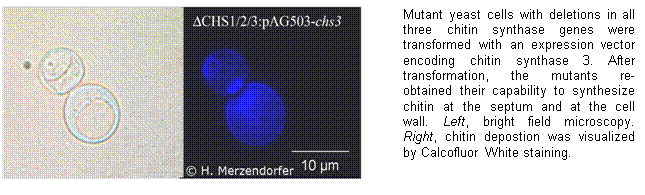
From our studies we have learned that we can not
completely understand the regulation of chitin synthases unless we do not
understand its maturation and intracellular trafficking. Fungi produce chitin
as a component of the cell wall. In budding baker’s yeast, Saccharomyces
cerevisiae, chitin synthase 3 is transported to the bud neck where it
produces a chitinous ring stabilizing the budding zone. To allow analyses of
chitin synthase maturation and trafficking, we genetically dissect involved
genes and biochemically analyze their functions.
Cited and recommended
literature:
Arakane, Y., Hogenkamp, D. G., Zhu, Y. C., Kramer, K. J., Specht, C. A.,
Beeman, R. W., Kanost, M. R. and Muthukrishnan, S. (2004). Characterization of two chitin synthase genes of the red
flour beetle, Tribolium castaneum, and alternate exon usage in one of
the genes during development. Insect Biochem. Mol. Biol. 34, 291-304.
Becker, B.
(1978). Effects of 20-hydroxy-ecdysone, juvenile hormone, Dimilin, and Captan on in vitro
synthesis of peritrophic membranes in Calliphora erythrocephala. J. Insect Physiol. 24: 529-533.
Bracker, C. E., Ruiz-Herrera, J. and Bartnicki-Garcia, S. (1976). Structure
and transformation of chitin synthetase particles (chitosomes) during microfibril synthesis in vitro.
Proc. Natl. Acad. Sci. USA 73: 4570-4574.
Cabib, E. (1994). Nomenclature of genes related to
chitin synthesis. Yeast Newslett. XLIII: 58.
Clarke, L. Temple, G. H. and Vincent, J. F. (1977) The
effects of a chitin inhibitor-dimilin- on the
production of peritrophic membrane in the locust, Locusta
migratoria. J. Insect Physiol. 23: 241-246.
Charnock, S.J. and Davies,
G.J. (1999) Structure of the nucleotide-diphospho-sugar
transferase, SpsA from
Bacillus subtilis, in native and nucleotide-complexed forms. Biochemistry, 38, 6380-6385.
Cohen, E. (1991). Chitin biochemistry. In Physiology of the insect epidermis (ed. K. Binnington
and A. Retnakaran), pp. 94-112.
Ibrahim, G. H., Smartt, C. T., Kiley, L. M. and Christensen, B. M. (2000). Cloning and characterization of a chitin synthase cDNA from the
mosquito Aedes aegypti. Insect Biochem. Mol.
Biol. 30: 1213-1222.
Gagou, M. E., Kapsetaki, M., Turberg, A. and Kafetzopoulos, D.
(2002). Stage-specific
expression of the chitin synthase DmeChSA and DmeChSB genes during the onset of Drosophila
metamorphosis. Insect Biochem. Mol. Biol. 32, 141-146
Coutinho, P.M., Deleury, E., et al. (2003) An
evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol., 328, 307-317.
Hogenkamp, D. G., Arakane,
Y., Zimoch, L., Merzendorfer, H., Kramer, K. J.,
Beeman, R. W., Kanost, M. R. and Muthukrishnan, S. (2005). Chitin
synthase genes in Manduca sexta: Characterization of a gut-specific
transcript and differential tissue expression of alternately spliced mRNAs
during development, Insect Biochem. Mol. Biol. 35, 529-540.
Ibrahim,
G. H., Smartt, C. T., Kiley,
L. M. and Christensen, B. M. (2000). Cloning and characterization of a chitin synthase cDNA from the
mosquito Aedes aegypti. Insect Biochem.
Mol. Biol. 30, 1213-1222.
Ishaaya,
Kramer, K. J., Hopkins, T. L. and Schaefer, J. (1995).
Applications of solids NMR to the analysis of insect
sclerotized structures. Insect Biochem.
Mol. Biol. 25: 1067-1080.
Lehane, M.
J. and Billingsley, P. F. (1996). Biology of the
insect midgut.
Lehane,
M. J. (1997). Peritrophic matrix structure and
function. Annu.
Rev. Entomol. 42, 525-550
Merzendorfer, H. and Zimoch, L. (2003). Chitin
metabolism in insects: structure, function and regulation of chitin synthases
and chitinases. J.
Exp. Biol. 206, 4393-4412.
Merzendorfer, H. (2006) Insect
chitin synthases: a review. J. Comp. Physiol. [B], 176, 1-15.
Merz, R. A., Horsch, M., Nyhlen,
L. E. and Rast, D. M. (1999). Biochemistry
of
chitin synthase.
EXS. 87: 9-37.
Moussian, B., Schwarz, H., et al. (2005) Involvement of chitin
in exoskeleton morphogenesis in Drosophila melanogaster. J Morphol,
264, 117-130.
Peters, W. (1992). Peritrophic
membranes. Zoophysiology Series Vol. 30.
Tellam, R. L. (1996). The peritrophic matrix. In Biology of the insect midgut (ed. M. J. Lehane
and P. F. Billingsley), pp. 86-114.
Tellam, R. L., Vuocolo, T., Johnson, S. E., Jarmey, J. and
Pearson, R. D. (2000). Insect chitin synthase cDNA
sequence, gene organization and expression. Eur. J. Biochem. 267:
6025-6043.
Wagner, G. P. (1994). Evolution and multi-functionality of
the chitin system. EXS. 69: 559-577.
Zimoch,
L. and Merzendorfer, H. (2002). Immunolocalization
of chitin synthase in the tobacco hornworm. Cell Tissue Res. 308, 287-297.