SERCA regulation by Peptides
Project Title: Functional characterization of a novel mechanism regulating SERCA activity and Ca2+ homeostasis in Drosophila melanogaster
Funding Source: DFG SFB944, TP21
Principle Investigator: Heiko Harten with Annika Buhr, Ronja Schiemann
I am using Drosophila melanogaster as a model organism to understand the physiological functionality of metalloproteases in general and of neprilysins in particular. In addition to investigating their functional relevance during development, we are interested in understanding the regulatory impact of neprilysins on distinct physiological processes, such as muscle and heart contraction or energy metabolism.
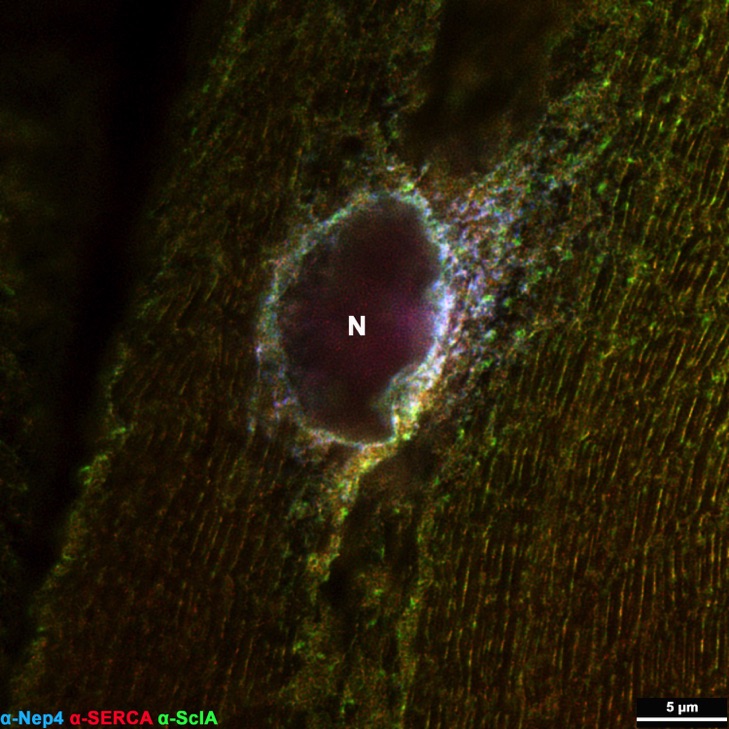
STED-based triple labeling of Nepilysin4, SERCA, and Sarcolamban in 3rd instar larval body wall muscles confirms co-localization of all factors in distinct membrane microcompartments of the sarcoplasmic reticulum, particularly around the nucleus (N).